Helo teman2 radiografer yang saya cintai beberpa hari yang lalu saya mendengar keluhan dari radiografer ada yang belum bisa melakukan pemeriksaan canggih.Nah dalam hati kemana aja bang ? tapi nggak begitu2 nya.Ternyata memang benar di beberapa Rumah sakit yang saya temui di Surabaya memang ada juga yang belum bisa mengoperasikan CT scan 64 Slice, MRI 1,5T 3D Angiografy,PET scan.Aduh memang mumet kalo belajar semua, kalo saya disuruh belajar semua mungkin ya BT he he he he ..
Nggak begitu kok.Tapi dari keluahan diatas saya mengambil kesimpulan bagaiman jika kita lupa atau kagak bisa so bertanya kepada siapa? nah untuk itulah kita perlu sarana sharing radiografer se Indonesia.Rasanya ini adalah ide yang cemerlang.Nah siapa yang mau mempelopori ? kalo gak ada ya saya aja lah gak apa 2.
Kemarin saya meluncurkan MILIS RADIOGRAFER bisa di klik di http://health.groups.yahoo.com/group/radiographer/
Nah dari ide ini saya menemukan banyak sekali masukan dari teman2 milis yang sangat bermanfaat.Nah kapan lagi kita berbagi ilmu radiology.Ilmu gak akan berkembang kalo gak ada yang tanya, betul gak???Nah kalo ada yang terus tanya maka ada yang terus cari jawaban.Nah ilmu pasti berkembang.
Sabtu, 26 Desember 2009
Jumat, 25 Desember 2009
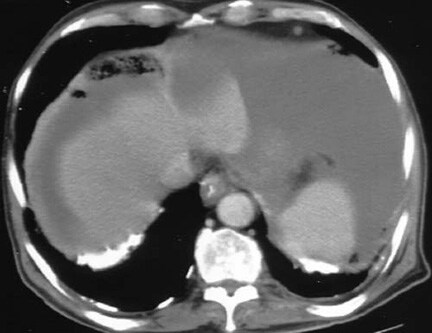
MRI ascites? banyak artifact...
ascites merupakan suatu akumulasi cairan didalam rongga abdomen, pada posisi supine cairan ini terkumpul pada bagian2 tertentu yaitu pelvis dan usus para colon.
Transdutat,bergerak bebas,merupakan kumpulan cairan sederhana
Membuat MRI abdomen dengan pasien ascites memang gampang2 susah.Kenapa ? karena saat pasien kita posisikan supine maka cairan akan tertumpuk di bagian posterior dari cavum abdomen,bukan hanya itu permasalahannya saat kita buat MRI irisan axial maka akan terlihat banyak artifact dari pergerakan cairan, walaupun sudah kita pasang respiratory gatting.Hal ini bukan ada kesalahan dari komputer Anda.
Tenang saja yang terpenting kita sebagai radiografer pinter2 mengakali membuatnya.Bagaimana solusinya ?
- Ascites hemorogiik : hal ini menunjukkan adanya keganasan pada peritonium,walaupun dapat merupakan perdarahan akibat trauma
- Ascites chilosa : chilus kadang2 mengumpul pada rongga peritonium penyebabbnya meliputi limfangiektasia kengenital,trauma abdomen termasuk pembedahan yang disertai kerusakan pada saluran limfatik abdomen,infiltrasi,keganasan,filariasi dan tuberkulosis.Gale Encyclopedia of Cancer: Ascites
Transdutat,bergerak bebas,merupakan kumpulan cairan sederhana
- Sirosis
- Hipoproteinemia
- Gagal ginjal
- Perikarditis
- Gagal jantung
- Sindrom Budd-Chiari
- Karsinoma primer atau sekunder
- Peritonitis tuberkulosis
- Pankratitis
- Syndrome Meig (tumor jinak ovarium dengan ascites)http://emedicine.medscape.com/article/255450-overview
Membuat MRI abdomen dengan pasien ascites memang gampang2 susah.Kenapa ? karena saat pasien kita posisikan supine maka cairan akan tertumpuk di bagian posterior dari cavum abdomen,bukan hanya itu permasalahannya saat kita buat MRI irisan axial maka akan terlihat banyak artifact dari pergerakan cairan, walaupun sudah kita pasang respiratory gatting.Hal ini bukan ada kesalahan dari komputer Anda.
Tenang saja yang terpenting kita sebagai radiografer pinter2 mengakali membuatnya.Bagaimana solusinya ?
- Tetap pakai respiratory (teknik breath-hold ) jika pasien tidak bisa pakai ( teknik trigger)
- Pasang rest lab atau saturasi
- Pakai teknik fat supprasion
- Fold aver direction ganti FH.
Rabu, 23 Desember 2009
Mailing list radiografer
Dear teman2 Radiografer yang saya cintai.hari ini telahaku terbitkan mailing list khus radiografer yang ingin berbagi dan sharing seputar ilmu Radiology sukur2 ilmu tentang MRI kebetulan kan web ini tentang MRI.Nah yang ingin bergabung silakan add email ini radiographer@yahoogroups.com
dan join ke member http://health.groups.yahoo.com/group/radiographer/
Ok kita ketemu di milis
Dear Ferry Indriasmoko Amd Rad
dan join ke member http://health.groups.yahoo.com/group/radiographer/
Ok kita ketemu di milis
Dear Ferry Indriasmoko Amd Rad
Minggu, 20 Desember 2009
MRI Infarct Cerebri
Penyakit vaskuler
Infarct cerebri
Buat teman2 radiografer Indonesia or mancanegara, sering kita mendengar istilah stroke ada yang bilang karena perdarah ada juga yang bilang karena penyempitan pembuluh darah, nah hari ini saya akan membuka ilmunya yang akan saya padu dengan teknik pembuatan MRI paket stroke.
Pada orang dewasa otak manusia beratnya hanya 2% dari berat badan,tetapimemerlukan oksigen 20% dari seluruh intake O2 dan 16% dari cardiac output.
Rekasinya begitu cepat apabila terjadikekurangan oksigen diotak,mula2 terjadi kerusakan reversible kemudian berlanjut menjadi irreversible.Infarct cerebri (serangan ischemic & encephalomalacia)menjadi penyebab kematian no.3 setelah serangan jantung dan tumor.
Yang mendasari terjadinya stroke adalah kecelakaan lalulintas yang menyebabkan terjadinya thrombosis arterii (75%) kasus emboli 10% kasus dan perdarahan massif (15%).Material thrombus akan menyebabkan penyempital lumen arteri cerebral dan akan menghambat aliran darah dan menyebbkan kekurangan oksigen dan energy ke otak.Trombus paling banyak terjadi pada arteri akibat dari arteriosclerotic didalam vaskuler pada pasien yang tua dan akibat keradangan pada pasien yang muda.
Sumber trombolitic adalah plaque extracranial atau ulcerasi pembuluh darah brachiocephalic,seperti juga pada gangguan jantung.
Secara histology infarct dibagi menjadi 3 fase:
- Pelunakan awal dengan intra / ekstra cellular
- Pada hari kedua terjadi degenerasi selubung myelin dan karyolsis neuroglia secara bersamaan terjadi pula phagocytosis myelin oleh sel granuler lemak (sel microglia dan histiocyt)
- Encephalomalacia
Jumat, 18 Desember 2009
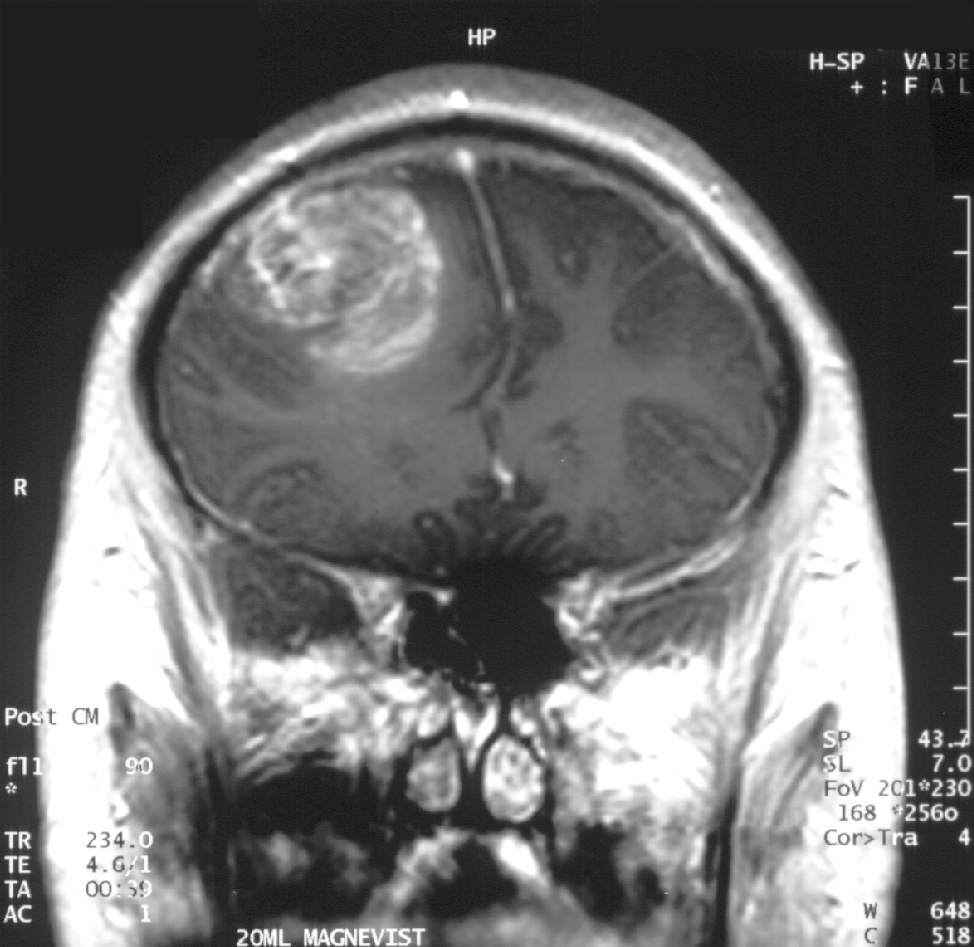
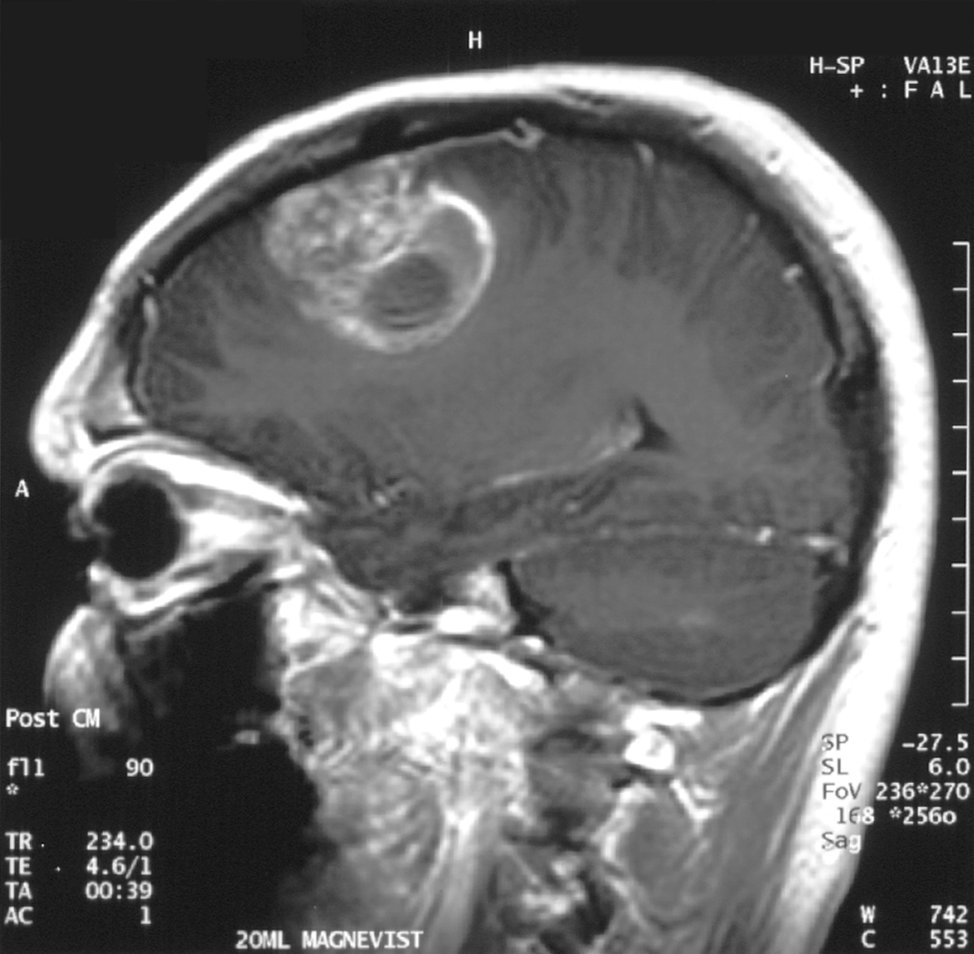
MRI Glioblastoma
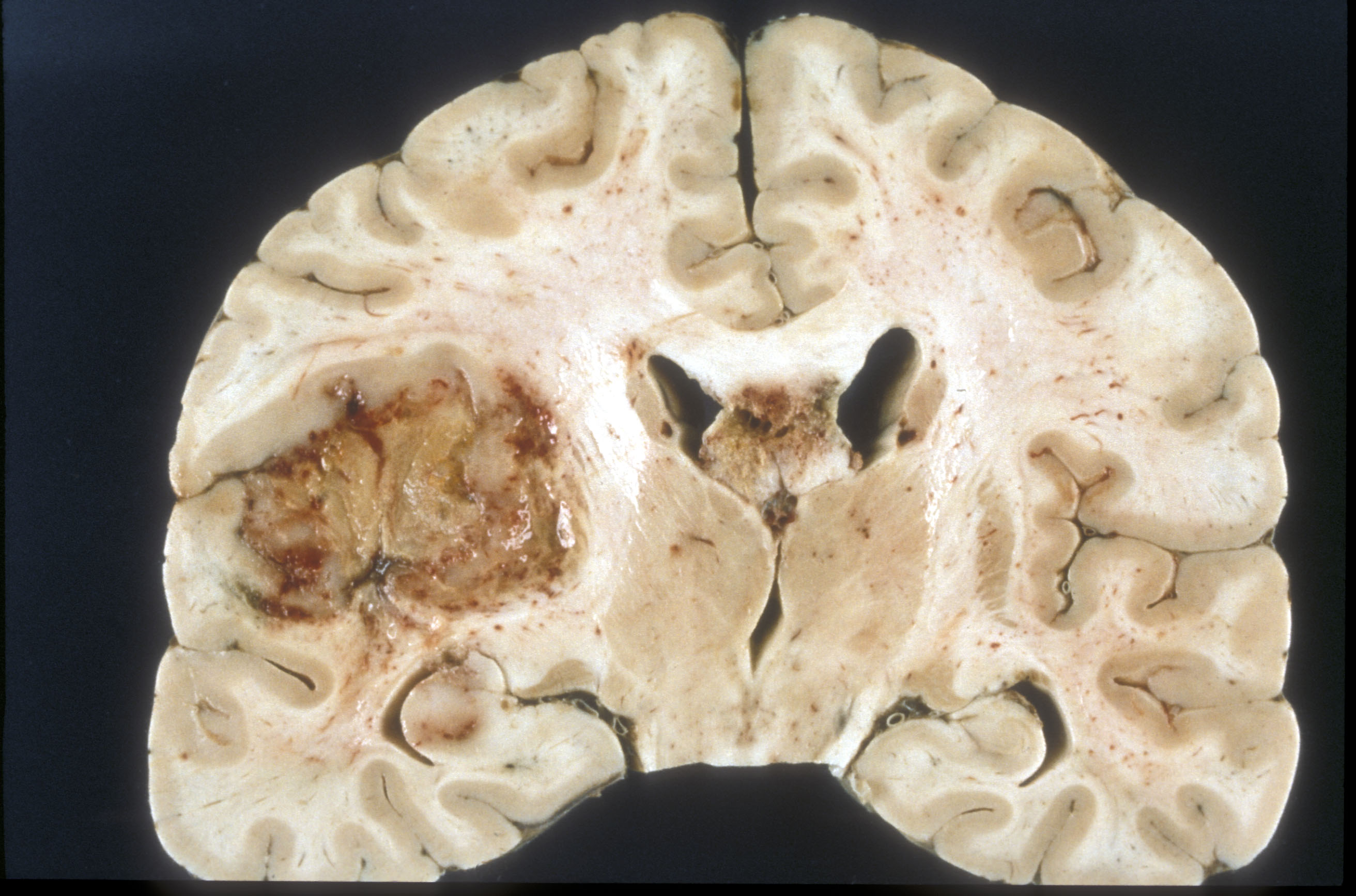 Glioblastoma adalah sebuah tumor sistem saraf pusat yang terbentuk dari glial jaringan otak dan sumsum tulang belakang dan sel sel yang terlihat sangat berbeda dengan jaringan yang normal.Glioblastoma biasanya terjadi pada orang dewasa dan mempengaruhi otak dari pada sumsum tulang belakangGlioblastoma:: Molecular Mechanisms of Pathogenesis and Current Therapeutic Strategies
Glioblastoma adalah sebuah tumor sistem saraf pusat yang terbentuk dari glial jaringan otak dan sumsum tulang belakang dan sel sel yang terlihat sangat berbeda dengan jaringan yang normal.Glioblastoma biasanya terjadi pada orang dewasa dan mempengaruhi otak dari pada sumsum tulang belakangGlioblastoma:: Molecular Mechanisms of Pathogenesis and Current Therapeutic StrategiesGlioblastoma adalah tumor brain yang sangat agresif type primer.52% dari kasus parenchym tumor dan 20% semua tumor intracranial.Glioblastoma terjadi dari 2-3 kasus per 100.000 orang di Amerika Utara dan Eropa.Menurut the WHO classification of the tumors of the central nervous system.
Nama standart untuk tumor ini adalah Glioblastoma, ada dua varian yaitu : giant cell glioblastoma and gliosarcoma .
Gejalanya biasanya kejang,muntah, nyeri kepala dan hemiparese.
Diagnosis jika dilihat dengan modalitas MRI maka akan terlihat ring-enhancing lesions,seperti gb yang diatas.Gambar MRI diatas terlihat jelas ring enhancment saat T1W post contrast.saat kita spectroscopy akan nampak metabolisme cholin sangat tinggi.
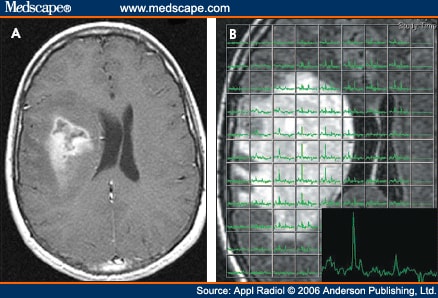 nampak pada gambar kanan bawah terlihat rasio cholin dan creatin yang tinggi.Menunjukkan agresifitas yang tinggi.
nampak pada gambar kanan bawah terlihat rasio cholin dan creatin yang tinggi.Menunjukkan agresifitas yang tinggi.Ada juga nampak tampilan yang tidak spesific sebagai lainnya seperti abses,abses, metastasis, tumefactive multiple sclerosis,
Glioblastoma sering terjadi pada laki2 walaupun alasanya belum jelas, biasanya tumor ini sangat sporadis tanpa kecenderungan genetik
Faktor resiko lainnya meliputi :
- Jenis kelamin ( laki2)
- Umur diatas 50 tahun
- Etnic : Latin dan Asia
- Salah satu penyebab kelaina genetic yang berkaitan Neurofibromatosis, tuberous sclerosis, Von Hippel-penyakit Lindau, Li-Fraumeni sindrom, sindrom Turcot

Kamis, 17 Desember 2009
MRI Bayi 1 bulan DD Toxoplasma
Teman2 radiografer sudah lama saya tidak mengisi di blog http://belajar-mri.blogspot.com/ kangen rasanya,lagi ada tugas menghitung unit cost biaya MRI.Nah hari ini saya akan bercerita tentang satu pasien bayi laki2 umur 1 bulan DD toxoplasma dimintakan MRI kepala dengan kontras.Riwayat pasien kejang 8x.Pasien datang dari NICU RS.Husada Utama Surabaya.
Pemeriksaan MRI ini kita memerlukan bantuan unit anatesi dan dokter anastesi.
Bagaimana prosedur Pemeriksaannya :
Pasien dianatesi terlebih dulu agar tidur kemudian kita posisikan supine kepala dimasukkan di head coil, pasang earpluq di kedua telinga,dokter anastesi dan perawat MRI standby di dalam ruang MRI menemani bayi.jangan lupa pasang oksigen.protokol yang kita pilih yaitu Brain bayi terdiri dari :
Pemeriksaan MRI ini kita memerlukan bantuan unit anatesi dan dokter anastesi.
Bagaimana prosedur Pemeriksaannya :
Pasien dianatesi terlebih dulu agar tidur kemudian kita posisikan supine kepala dimasukkan di head coil, pasang earpluq di kedua telinga,dokter anastesi dan perawat MRI standby di dalam ruang MRI menemani bayi.jangan lupa pasang oksigen.protokol yang kita pilih yaitu Brain bayi terdiri dari :
- Survey_MST
- Ref_SHC_8
- T2W_TSE axial,coronal,Sagital
- T1W_SE
- DW_SSh_og
- T2Flair
- T1W Post contrast
Selasa, 08 Desember 2009
Saatnya...
Hari ini hari ketiga setelah senin ya betul lah karena ini hari rabu,Apa maksudnya /? udah gak perlu dibahas.Emang ada apa di hari Rabu.Gak tahu nih ini hari kok aku lagi gak semangat soalnya pasien MRI hanya 2 pasien, sedih rasanya.Kira2 seorang radiografer bisa nggak menambah pasien ? aduh ngapai fer susah susah kan enak gak ada pasien!.
Nah ini nih pikran orang yang gak mau maju
Saatnya kita belajar membuat MRI yang baik.Beberapa minggu yang lalu rasanya saya sudah mebuat tulisan tentang TIPS Parameter MRI.beberapa waktu yang lalu ada teman saya yang bertanya bagaiman membuat MRI prostat yang bagus gambarnya? "kata temanku begitu " maksudnya?"saya tanya balik" pada saat dia membuat banyak artefak yang terjadi.
Ok let's go kita amati dan lihat.
Coba kita akan buat gambar prostat pada bidang axial, survey nya buat dulu sagital biar gampang melihat organ prostatnya
Lihat gambar disamping:
saturasi yang atas digunakan untuk saturasi vessel yang ventral depan di buat untuk saturasi fat / lemak.Baru buat irisan tipis di daerah prostat.Satu lagi alangkah lebih baiknya gak pake kontras air yang melalui anus.
apakah sudah bisa dimenegrti teman2
Nah ini nih pikran orang yang gak mau maju
Saatnya kita belajar membuat MRI yang baik.Beberapa minggu yang lalu rasanya saya sudah mebuat tulisan tentang TIPS Parameter MRI.beberapa waktu yang lalu ada teman saya yang bertanya bagaiman membuat MRI prostat yang bagus gambarnya? "kata temanku begitu " maksudnya?"saya tanya balik" pada saat dia membuat banyak artefak yang terjadi.
Ok let's go kita amati dan lihat.
Coba kita akan buat gambar prostat pada bidang axial, survey nya buat dulu sagital biar gampang melihat organ prostatnya
Lihat gambar disamping:
saturasi yang atas digunakan untuk saturasi vessel yang ventral depan di buat untuk saturasi fat / lemak.Baru buat irisan tipis di daerah prostat.Satu lagi alangkah lebih baiknya gak pake kontras air yang melalui anus.
apakah sudah bisa dimenegrti teman2
Patient Safety
The main aspect of patient safety in any MRI facility is magnetic safety.It is essensial that all patient, relatives, and other medical or non-medical! personnel are prevented from entering the magnetic field until they have been properly screened.Phisical barriers, such as doors and large warning sign, are common ways of achieving this.Clerical personnel ( who are ussually situated at the entrence to the unit) should be aware of who is present in the facility an wheter they have been checked for magnetic safety.Thorough screening of each patient and anyone who is to enter the field is extremely important.Failure to do so may result in injury or even death.All centres should have a proper screening pilicy which includes checking for :
- Pacemaker
- Aneurysma clips
- Intra-ocular foreign bodies
- Metal device or prosthesis
- Cochlear implan
- Possibility of early pregnancy
- Removal of all jewellery, credit cards,watches,etc
Minggu, 29 November 2009
TIPS parameter MRI
Hallo teman2 radiografer hari ini saya akan memberikan TIPS bagaimana membuat gambar MRI jadi ciamik.Semoga Anda sudah mengerti istilah - istilah di MRI.Ok kita mulai
hal penting pertama
1.Bagaimana jika kita menaikkan TR apa yang terjadi?
Keuntungan:
Keuntungan :
Keuntungan :
Keuntungan ;
Keuntungan :
Keuntungan :
Keuntungan :
hal penting pertama
1.Bagaimana jika kita menaikkan TR apa yang terjadi?
Keuntungan:
- SNR meningkat
- Number of slice juga meningkat
- Scan time meningkat
- T1 weight menurun
Keuntungan :
- Scan time lebih cepat
- T1 weight meningkat
- SNR jadi turun
- Number of slice turun
Keuntungan :
- T2 weight juga meningkat
- SNR jadi menurun Jika TE kita turunkan maka sebaliknya yang terjadi
Keuntungan ;
- SNR naik di semua jaringan
- Bagusnya lagi menurunkan flow artifact
- Menurunkan spasial resolution dan partial volume didalam slice
Keuntungan :
- Spasial resolution jadi meningkat tapi menurunkan partial volume di dalam slice selection
- Menurunkan SNR di semua jaringan
- Yang pasti menurunkan cover of anatomy ya nggak
Keuntungan :
- Spatial resolution nya pasti naik
- SNR pasti turun
Keuntungan :
- SNR naik di semua jaringan
- Yang pasti resolusinya turun dan image gak bagus
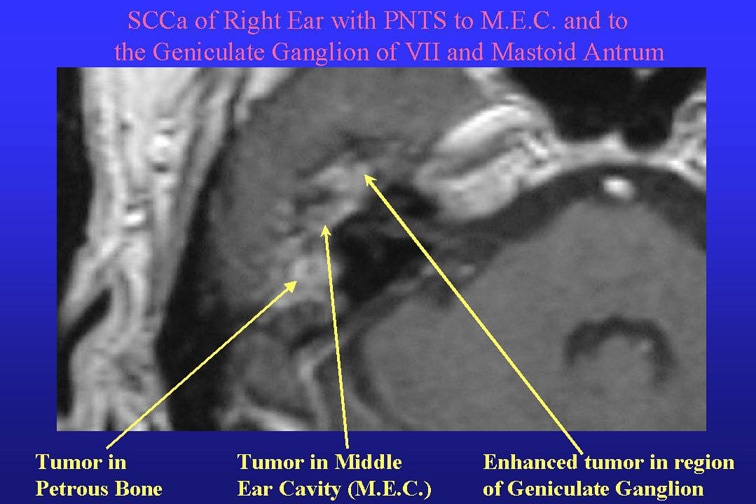
Facial Nerve dan Masalah Bell's Palsy
Apa saraf wajah itu (nerve VII)?
Saraf wajah adalah saraf yang mengendalikan otot2 wajah.Hal ini memungkinkan untuk berekspresi,tersenyum, menangis dan mengedipkan mata.Cedera pada saraf wajah menyebabkan bencana sosial dan psikologis cacat fisik.Pengobatan mungkin memerlukan rehabilitasi yang luas artinya tidak mudah.
Apa gejala gejala saraf wajah ?
Masalah sarah wajah bisa mengakibatkan kelumpuhan otot wajah ataupun kelemahan otot wajah sehingga menyebabkan salah satu wajah terlihat miring.
Kondisi apa saja yang mempengaruhi saraf wajah?
Ada banyak penyebab gannguan saraf wajah antara lain:
1.Trauma; trauma kelahiran,patah tulang tengkorak,luka wajah,telingan tengah cedera.
2.Penyakit sistem saraf.Millard-Gubler sindrom
3.Infeksi dari telinga atau wajah (Ramsey-Hunt syndrome).
4.Metabolic diabete militus
5.Tumor; akustik neuroma
6.Bell's Palsy atau kelumpuhan saraf wajah
Pemeriksaan penunjang untuk pemeriksaan Hemifacial spasme adalah MRI.MRI sebagai modalitas unggul bisa mengetahui atau mendiagnosa letak atau kelainan saraf ke-7
Saraf wajah adalah saraf yang mengendalikan otot2 wajah.Hal ini memungkinkan untuk berekspresi,tersenyum, menangis dan mengedipkan mata.Cedera pada saraf wajah menyebabkan bencana sosial dan psikologis cacat fisik.Pengobatan mungkin memerlukan rehabilitasi yang luas artinya tidak mudah.
Apa gejala gejala saraf wajah ?
Masalah sarah wajah bisa mengakibatkan kelumpuhan otot wajah ataupun kelemahan otot wajah sehingga menyebabkan salah satu wajah terlihat miring.
Kondisi apa saja yang mempengaruhi saraf wajah?
Ada banyak penyebab gannguan saraf wajah antara lain:
1.Trauma; trauma kelahiran,patah tulang tengkorak,luka wajah,telingan tengah cedera.
2.Penyakit sistem saraf.Millard-Gubler sindrom
3.Infeksi dari telinga atau wajah (Ramsey-Hunt syndrome).
4.Metabolic diabete militus
5.Tumor; akustik neuroma
6.Bell's Palsy atau kelumpuhan saraf wajah
Pemeriksaan penunjang untuk pemeriksaan Hemifacial spasme adalah MRI.MRI sebagai modalitas unggul bisa mengetahui atau mendiagnosa letak atau kelainan saraf ke-7
Bagaimana membuat gambar MRI kasus Hemifacial spasme?
Yang pertama kita lakukan adalah membuat irisan rutin untuk Brain teknik yang meliputi T2W_TSE axial,coronal dan sagital,T1w_SE axial ditambah T2W_FLAIR dan DWI.
Selanjutnya buat irisan 3D teknik sejajar telinga T2 kalo di Philips disebut T2 DRIVE HR akan menghasilkan gambaran 3D slices 100 tanpa Gap.Kita juga bisa membuat irisan MPR dan data akuisis tadi bisa dilihat dari coronal dan sagital.
Ok teman2 radiografer kita sudah mngerti bagaimana membuat irisan 3D untuk hemifacial spasme
Jumat, 27 November 2009
Total Cervical Disc Replacement
Materi ini ditulis oleh M.Sofyanto dr.SpBS Beliu salah satu dokter spesialis bedah saraf di RS.Spesialis Husada Utama Surabaya.
Ganti sendi leher,metode baru untuk spondilosis leher
Banyak yang tidak menyadari kalau sendi leher (bantalan diskus) melakukan pergerakan paling banyak dibanding sendi seluruh tubuh,sehingga berpeluang terjadi kerusakan atau degenerasi lebih awal,data terbaru menyatakan 50% diatas 50th mengalami leher dan bahu sebagai keluahan paling menonjol.
Gejala ini disebabkan penekanan saraf sumsum leher akibat dari bantalan diskus yang lepas,Penebalan ligamnet pelindung saraf dan pengapuran tulang leher yang menusuk akar saraf.
Keluhan ini meliputi :
a. Nyeri dileher seperti menusuk dan terbakar yang menyiksa terutama malam hari berkurang ketika leher digerakkan atau saat bekerja,bahkan nyeri mejalar sampai sekitar telinga dan mata.
b. Nyeri kepala bisa satu sisi kadang disertai migrain dan vertigo
c. Nyeri bahu satu atau dua sisi pada puncak bahu mencengkram keras,kadang nyeri sampai ke dada
d. Nyeri lengan menjalar sampai tangan, kesemutan dan kelemahan jari hingga sulit memegang barang ataupun menulis.
e. Gannguan jalan bila keadaan semakin berat disertai jalan kaku dan berat kadang nyeri sperti sengatan listrik di tangan dan kaki.
Sebagian besar penyakit ini tidak memerlukan operasi dan sembuh spontan dengan penanganan yang baik, selama tagun 2008 terdapat 352 pasien spondilosis leher di RS.Spesialis Husada Utama Surabaya,hanya 52 kasus yang menjalani operasi.indikasidilakukan operasi sangat ketat bila pengobatan tidak menolong.
Metode terbaru penggantian sendi leher
Teknik operasi mikrosurgery memudahkan semua tindakan ooperasi leher tanpa resiko yang berarti dan aman, melalui kulit leher depan dua setengah centimetr dengan bantuan mikroskop khusus bisa memisahkan organ vital disekitar leher seperti arteri karotis,laring,usofagus, disisihkan.selama operasi keluarga menyaksikan lewat monitor dan bekomunikasi langsung dengan dokter.
Melewati celah ruas tulang leher semua penekanan dan penjepitan dan pengapuran dibebaskan dan bantalan ruas tulang leher yang rusak diganti dengan bantalan baru yang berfungsi seperti yang asli, bergerak ke suemua arah dan mampu menerima beban sebagaimana leher normal.
Operasi ini dikenal sebagai TOTAL CERVICAL DISC REPLACEMENT menggunakan bantalan discus sperti aslinya yang disebut cervical mobile prothesis, segera setalah operasi pasien dibangunkan dan boleh menngerakkan leher tanpa penyangga leher (collar brace) diperbolehkan pulang esok harinya setelah makan pagi dan tidak ada keluhan yang berarti.
Ganti sendi leher,metode baru untuk spondilosis leher
Banyak yang tidak menyadari kalau sendi leher (bantalan diskus) melakukan pergerakan paling banyak dibanding sendi seluruh tubuh,sehingga berpeluang terjadi kerusakan atau degenerasi lebih awal,data terbaru menyatakan 50% diatas 50th mengalami leher dan bahu sebagai keluahan paling menonjol.
Gejala ini disebabkan penekanan saraf sumsum leher akibat dari bantalan diskus yang lepas,Penebalan ligamnet pelindung saraf dan pengapuran tulang leher yang menusuk akar saraf.
Keluhan ini meliputi :
a. Nyeri dileher seperti menusuk dan terbakar yang menyiksa terutama malam hari berkurang ketika leher digerakkan atau saat bekerja,bahkan nyeri mejalar sampai sekitar telinga dan mata.
b. Nyeri kepala bisa satu sisi kadang disertai migrain dan vertigo
c. Nyeri bahu satu atau dua sisi pada puncak bahu mencengkram keras,kadang nyeri sampai ke dada
d. Nyeri lengan menjalar sampai tangan, kesemutan dan kelemahan jari hingga sulit memegang barang ataupun menulis.
e. Gannguan jalan bila keadaan semakin berat disertai jalan kaku dan berat kadang nyeri sperti sengatan listrik di tangan dan kaki.
Sebagian besar penyakit ini tidak memerlukan operasi dan sembuh spontan dengan penanganan yang baik, selama tagun 2008 terdapat 352 pasien spondilosis leher di RS.Spesialis Husada Utama Surabaya,hanya 52 kasus yang menjalani operasi.indikasidilakukan operasi sangat ketat bila pengobatan tidak menolong.
Metode terbaru penggantian sendi leher
Teknik operasi mikrosurgery memudahkan semua tindakan ooperasi leher tanpa resiko yang berarti dan aman, melalui kulit leher depan dua setengah centimetr dengan bantuan mikroskop khusus bisa memisahkan organ vital disekitar leher seperti arteri karotis,laring,usofagus, disisihkan.selama operasi keluarga menyaksikan lewat monitor dan bekomunikasi langsung dengan dokter.
Melewati celah ruas tulang leher semua penekanan dan penjepitan dan pengapuran dibebaskan dan bantalan ruas tulang leher yang rusak diganti dengan bantalan baru yang berfungsi seperti yang asli, bergerak ke suemua arah dan mampu menerima beban sebagaimana leher normal.
Operasi ini dikenal sebagai TOTAL CERVICAL DISC REPLACEMENT menggunakan bantalan discus sperti aslinya yang disebut cervical mobile prothesis, segera setalah operasi pasien dibangunkan dan boleh menngerakkan leher tanpa penyangga leher (collar brace) diperbolehkan pulang esok harinya setelah makan pagi dan tidak ada keluhan yang berarti.
Siringomielia
Ok teman2 udah lama nih aku gak nulis di blog kesayanganku ini,Habis sibuk membantu acara pernikahan kakak istriku weleh weleh weleh....Hari ini kita akn belajar tentang yang namanya Siringomieli yang biasanya kita temukan saat MRI cervikal.Oklangsung aja apa siringomielia itu?
Siringomielia adalah kavitas di dalam medula spinalis dan sering berhubungan dengan kanalis sentralis paling banyak ditemukan pada medula servikalis dan saat meluas ke batang otak disebut sebagai siringobulbia.Jalur nyeri dan temperatur menjadi terganggu dan dapat menimbulkan neuropathi sendi.
Gambaran Radiologisnya : MRI akan memperlihatkan cairan sentral yang mengisi kavitas.
Siringomielia adalah kavitas di dalam medula spinalis dan sering berhubungan dengan kanalis sentralis paling banyak ditemukan pada medula servikalis dan saat meluas ke batang otak disebut sebagai siringobulbia.Jalur nyeri dan temperatur menjadi terganggu dan dapat menimbulkan neuropathi sendi.
Gambaran Radiologisnya : MRI akan memperlihatkan cairan sentral yang mengisi kavitas.
Penyebab :
1.Kongenital berhubungan dengan malformasi chiari
2.Pascatrauma :akibat cidera medula spinalis
3.Pascainflamasi : setelah inveksi dan perdarahan subarahnoid
ada teori yang juga mengatakna tentang siringomielia adalah kista sumsum tulang belakan
Modalitas apa saja yang bisa menjadikan atau yang bisa menunjukkan penyakit ini :
- Magnetic resonance imaging (MRI)
- Electromyography (EMG)
- Lumbar puncture
- Computed tomography (CT scan)
- Myelogram.
When the spinal cord is damaged, symptoms of syringomyelia can include:
- Chronic severe pain, weakness, and stiffness in the back, shoulders, arms, or legs
- Headaches
- Loss of ability to feel extremes of hot or cold, especially in the hands
- Loss of bowel or bladder function
- Sweating problems
- Loss of sexual function.
Senin, 23 November 2009
Hemiparese? hati2 Stroke
Halo teman2 radiografer udah lama nih aku gak ngisi blogku udah kangen nih opingin shering lagi.Nah pagi ini kita belajar tentang MRI paket Stroke.Apa ada itu ? ya adalah kalo gak ada ya di ada adain hehehehehhe.Nah kebetulan pagi ini aku sudah dihadapkan oleh pasien dengan indikasi hemiparese sinistra dimintakan oleh dokter ahli saraf untuk MRI brain.Bgaimana cara membuatnya ? mari ikuti langkah2 berikut ini:
yang pertama adalah screening dulu pasiennnya ( sudah pernah sya jelaskan di bab sebelumnya beserta lembar screeningnya so saya gak perlu repot jelaskan kembali).Hal yang paling penting yang kedua adalah persilahkan pasien untuk kencing terloebih dahulu biar nanti kagak minta pipis saat pemeriksaan.
Berikut adalah studi kasusnya:
Laki2 61 tahun riwayat stroke 2005 tiba2 pelo indikasi hemiparese sinistra.dilakukan MRI Brain + MRA.
Teknik pemeriksaan :
Coil yang kita gunakan sense head coil 8 chanel.Buat survey terlebih dulu untuk acuan pembuatan irisan axial,sagital dan coronalnya.
Parameter yang kita pilih antara lain:
Survey_MST
Ref_SHC_8
T2W_TSE Axial
T2W_TSE Sagital
T2W_TSE Coronal
T2W_Flair Axial
T1W_SE Axial
DW_Ssh_og
T2 star_FFE
Slice thikness : 5mm gap :1 (20% of slice thickness)
Fold over direction : RL
ditambah teknik MRA TOF dengan slices : 150 covered full brain.
teknik T1W_TSE
TR : 450-600 TE:12-25
teknik T2W_TSE
TR : 3500-4500 TE : 100
teknik T2W_Flair
TR : 9000 TE : 120 TI : 2300
hasil MRI
yang pertama adalah screening dulu pasiennnya ( sudah pernah sya jelaskan di bab sebelumnya beserta lembar screeningnya so saya gak perlu repot jelaskan kembali).Hal yang paling penting yang kedua adalah persilahkan pasien untuk kencing terloebih dahulu biar nanti kagak minta pipis saat pemeriksaan.
Berikut adalah studi kasusnya:
Laki2 61 tahun riwayat stroke 2005 tiba2 pelo indikasi hemiparese sinistra.dilakukan MRI Brain + MRA.
Teknik pemeriksaan :
Coil yang kita gunakan sense head coil 8 chanel.Buat survey terlebih dulu untuk acuan pembuatan irisan axial,sagital dan coronalnya.
Parameter yang kita pilih antara lain:
Survey_MST
Ref_SHC_8
T2W_TSE Axial
T2W_TSE Sagital
T2W_TSE Coronal
T2W_Flair Axial
T1W_SE Axial
DW_Ssh_og
T2 star_FFE
Slice thikness : 5mm gap :1 (20% of slice thickness)
Fold over direction : RL
ditambah teknik MRA TOF dengan slices : 150 covered full brain.
teknik T1W_TSE
TR : 450-600 TE:12-25
teknik T2W_TSE
TR : 3500-4500 TE : 100
teknik T2W_Flair
TR : 9000 TE : 120 TI : 2300
hasil MRI
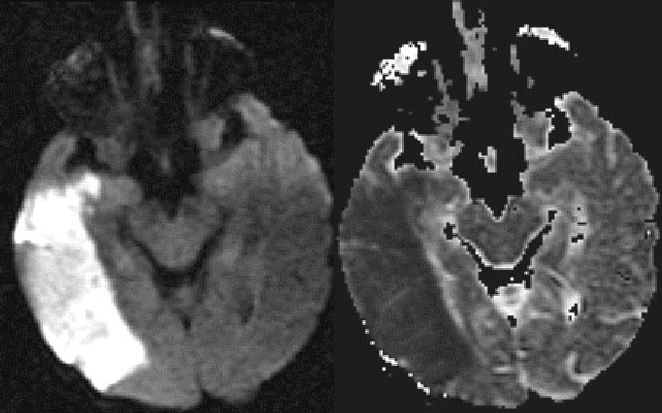
Terlihat digambar diatas DWi nampak area Bright menunjukkan acute stroke.
figure.MRA TOF
Jumat, 20 November 2009
MRI Breast RS.Husada Utama
MRI breast adalah salah satu modalitas canggih untuk mendiagnosa kelaian pada payudara.Modalitas ini mempunyai banyak keunggulan dibanding dengan modalitas yang lain terutama mamografi.MRI ini bukan untuk menggantikan modalitas yang lain termasuk USG dan mamografi.MRI Breast sebagai sarana diagnosa banding yang akurat.
MRI Breast Imaging dianjurkan bagi :
1. Wanita dengan riwayat keluarga menderita kanker payudara.
2. Wanita dengan hasil mammogram/ultrasound menunjukan adanya kelainan atau atas anjuran dokter.
3. Wanita diatas 40 tahun (dianjurkan memeriksakan diri setiap 2 tahun sekali).
4. Wanita yang menggunakan implant.
Tahun 1991 FDA (US food an drug administration )menyetujui modalitas ini untu screening / diagnosa kanker payudara.MRI ini sangat baik juga dalam kasus implan payudara yang tidak bisa dinilai di mamografi konvensional,Karena dapat menunjukkan gambaran jaringan yang jauh lebih detail.MRI juga berguna untuk staging kanker payudara, menentukan perawatan yang paling sesuai dan untuk mendiagnosa post teraphy.




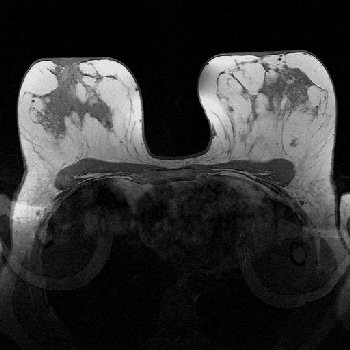


Recomendasi Biopsy
MRI Breast Imaging dianjurkan bagi :
1. Wanita dengan riwayat keluarga menderita kanker payudara.
2. Wanita dengan hasil mammogram/ultrasound menunjukan adanya kelainan atau atas anjuran dokter.
3. Wanita diatas 40 tahun (dianjurkan memeriksakan diri setiap 2 tahun sekali).
4. Wanita yang menggunakan implant.
Tahun 1991 FDA (US food an drug administration )menyetujui modalitas ini untu screening / diagnosa kanker payudara.MRI ini sangat baik juga dalam kasus implan payudara yang tidak bisa dinilai di mamografi konvensional,Karena dapat menunjukkan gambaran jaringan yang jauh lebih detail.MRI juga berguna untuk staging kanker payudara, menentukan perawatan yang paling sesuai dan untuk mendiagnosa post teraphy.
Jika kita lihat gambar diatas perbedaan jaringan payudara dan implan sangat jelas jika menggunakan mamografi konvensional hal ini tidak akan terlihat bagus, banyak artefact yang mengaburkan gambar.
Teknik pemeriksaan MRI payudara :
1.Pasien datang tidak perlu puasa.
2.Melepas barang2 yang mengandung logam.
3.Mempersilahkan mengganti baju pasien yang telah disediakan
4.Pasien masuk ruang MRI diposisikan Prone dengan payudara dibiarkan menggantung (tidak boleh nempel pada table)
5.Beri tahu pasien agar tidak boleh bergerak selama pemeriksaan akan mengakibatkan gambar yang akan dihasilkan kabur.
6.Informasikan pasien tetap rilex sambil mendengarkan musik.




Parameter yang kita gunakan :
1.sT1W_TSE
2.sT2W_TSE
3.STIR_fat sat
4.THRIVE_HR (T1 High Resolution Isotropic Volume Examination) using SPAIR / fat suppresion
Slice thicknes :4mm Gap 0-20% (0-0,6 mm)Fold over direction RL.
STUDI KASUS
Female 36 Th dengan indikasi skin dimpling mamma kanan.dikerjakan MRI Breast irisan axial coronal dan sagital dilanjutkan dengan Dynamic contrast.Jika perlu di tambah teknik DWIBS
History pasien : Post MRM + TRAM flap Ca mamma kanan 2007



Hasil diatas menunjukkan :
Tampak heterogenous enhanced focci pada mamma kanan kwadran lateral : pola kinetik curve menunjukkan early contrast uptake yang sama dengan bagian lain (kontrol) dan tidak terjadi early washout seperti lesi yang malignant.Batas lesi tampak ill define,tidak jelas adanya massa.
Selasa, 17 November 2009
MR IVP / MR Urography
Tulisan ini saya kutip dari web
http://radiographics.rsna.org/content/28/1/23.full
Introduction
A variety of techniques have been developed for imaging the urinary tract. Of these techniques, only two—computed tomographic (CT) urography and magnetic resonance (MR) urography—have the potential to provide a comprehensive assessment of the urinary collecting system, renal parenchyma, and surrounding structures. Although CT urography is nearing its potential in terms of spatial resolution, tissue differentiation, and elucidation of the renal anatomy, MR urography is a more nascent technology. MR urography is an evolving group of techniques with the potential to noninvasively provide the most comprehensive and specific imaging test available for many urinary tract abnormalities without the use of ionizing radiation (1,2). At the same time, formidable limitations and challenges remain for MR urography, including its relative insensitivity for renal calculi, relatively long imaging times, sensitivity to motion, and lower spatial resolution compared with CT and radiography. In this article, we review the most common MR imaging techniques used to image the urinary tract and discuss special considerations (pediatric patients, pregnant patients, renal insufficiency, imaging at 3 T) related to MR urography. In addition, we discuss and illustrate potential clinical applications of MR urography with respect to urolithiasis, urinary tract obstruction unrelated to urolithiasis, hematuria, congenital anomalies, and pre- and postoperative assessment. We also describe various pitfalls and artifacts associated with this modality.
MR Urographic Techniques
The most common MR urographic techniques used to display the urinary tract can be divided into two categories: (a) static-fluid MR urography (also known as static MR urography, T2-weighted MR urography, or MR hydrography), and (b) excretory MR urography (also known as T1-weighted MR urography) (1,3,4).
Static-Fluid MR Urography
T2-weighted techniques were the first clinically relevant means of visualizing the urinary tract with MR imaging (5–10). Static-fluid MR urography treats the urinary tract as a static column of fluid, using one of a variety of T2-weighted sequences that exploit the long T2 relaxation time of urine (11). Therefore, static-fluid MR urographic techniques closely resemble those used for T2-weighted MR cholangiopancreatography. Breath-hold T2-weighted MR urograms can be obtained with either thick-slab single-shot fast spin-echo techniques or similar thin-section techniques (eg, half-Fourier rapid acquisition with relaxation enhancement, single-shot fast spin-echo, single-shot turbo spin-echo). The signal intensity of background tissues can be adjusted by modifying the echo time or using fat suppression. Three-dimensional (3D) respiratory-triggered sequences can be used to obtain thin-section data sets that can then be postprocessed to create volume-rendered (VR) or maximum-intensity-projection (MIP) images of the entire urinary tract (11,12). Heavily T2-weighted static-fluid MR urograms resemble conventional excretory urograms and are useful for quickly identifying the level of urinary tract obstruction. However, identifying the cause of obstruction often requires additional sequences (Fig 1) (8). Static-fluid MR urography does not require the excretion of contrast material and is therefore useful for demonstrating the collecting system of an obstructed, poorly excreting kidney (10).
Figure 1a. Prostate cancer metastatic to lymph nodes in a 53-year-old man. (a) Coronal static-fluid MR urogram shows obstruction of the right distal ureter (arrow). (b) Coronal single-shot fast spin-echo image shows that an enlarged prostate gland and a metastatic lymph node (arrow) are responsible for obstruction of the ureter.
Figure 1b. Prostate cancer metastatic to lymph nodes in a 53-year-old man. (a) Coronal static-fluid MR urogram shows obstruction of the right distal ureter (arrow). (b) Coronal single-shot fast spin-echo image shows that an enlarged prostate gland and a metastatic lymph node (arrow) are responsible for obstruction of the ureter.
Static-fluid MR urograms can be obtained with single-shot fast spin-echo techniques in 1–2 seconds, which allows multiple images to be obtained sequentially in a relatively short period of time and played as a cine loop (13). Such image series ensure that both ureters are distensible along their entire lengths and that no fixed narrowings or standing columns exist (Fig 2; see also Movie 1 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). Cine MR urography is particularly helpful in confirming the existence of urinary tract stenosis (13). When acquiring a series of static-fluid MR urograms to be viewed in cine mode, one should allow 5–10 seconds between acquisitions to prevent radiofrequency saturation of the tissues, which causes progressive signal intensity loss on the images. Because cine MR urography is quick and easy to perform, we have made it a routine part of our MR urography protocol.
Figure 2a. Importance of cine MR urography in demonstrating the entire ureters with static-fluid techniques. (a) On a coronal thick-slab MR urogram from a cine series obtained in a 52-year-old woman with hematuria, the ureters are poorly delineated. (b) Coronal thick-slab MR urogram from the same series shows improved delineation of the ureters (arrows). Obtaining multiple sequential thick-slab images can be useful in demonstrating nondistended ureters.
Figure 2b. Importance of cine MR urography in demonstrating the entire ureters with static-fluid techniques. (a) On a coronal thick-slab MR urogram from a cine series obtained in a 52-year-old woman with hematuria, the ureters are poorly delineated. (b) Coronal thick-slab MR urogram from the same series shows improved delineation of the ureters (arrows). Obtaining multiple sequential thick-slab images can be useful in demonstrating nondistended ureters.
The T2 shortening effect of gadolinium prevents successful application of static-fluid MR urography during the excretory phase after the intravenous administration of gadolinium-based contrast material (Fig 3). Because static-fluid MR urography depends on the presence of urine within the collecting systems rather than the excretory function of the kidneys, it is ideally suited for patients with dilated, obstructed collecting systems (Fig 4; see also Movie 2 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). For patients with nondilated systems, the use of hydration, diuretics, or compression may enhance the quality of MR urography (9). Normal and abnormal fluid-filled structures can interfere with static-fluid MR urography, since the T2-weighted techniques used to display the urinary tract are not specific for urine. For this reason, intravenous hydration may be preferable to oral hydration prior to static-fluid MR urography in patients with nondistended ureters. Alternatively, acquisition planes or postprocessing reconstruction volumes can be adjusted to exclude bowel or other fluid-containing structures. At our institution, we do not use compression during MR urography.
Figure 3. Effect of gadolinium excretion on T2-weighted MR urographic techniques. Coronal excretory phase single-shot fast spin-echo MR image obtained through the kidneys after the intravenous administration of gadolinium-based contrast material shows low-signal-intensity urine (arrows) related to the T2 shortening effects of excreted gadolinium-based contrast material.
Figure 4a. Value of static-fluid MR urography in the setting of high-grade obstruction. (a) Thick-slab MR urogram obtained in a 64-year-old man with obstruction of the right ureteropelvic junction (UPJ) clearly shows dilatation of the right renal collecting system and the level of obstruction (arrow). Acquisition time for this image was approximately 2 seconds. (b) Coronal MIP image from excretory urographic data fails to show the right collecting system, despite an imaging delay of 5 minutes after the administration of gadolinium-based contrast material (acquisition time ≈ 20 seconds).
Figure 4b. Value of static-fluid MR urography in the setting of high-grade obstruction. (a) Thick-slab MR urogram obtained in a 64-year-old man with obstruction of the right ureteropelvic junction (UPJ) clearly shows dilatation of the right renal collecting system and the level of obstruction (arrow). Acquisition time for this image was approximately 2 seconds. (b) Coronal MIP image from excretory urographic data fails to show the right collecting system, despite an imaging delay of 5 minutes after the administration of gadolinium-based contrast material (acquisition time ≈ 20 seconds).
Excretory MR Urography
Excretory MR urography is roughly analogous to CT urography and conventional intravenous urography. A gadolinium-based contrast agent is administered intravenously, and the collecting systems are imaged during the excretory phase. Gadolinium shortens the T1 relaxation time of the urine, allowing the urine to initially appear bright on T1-weighted images. At standard doses of 0.1 mmol/kg, gadolinium-based contrast material quickly becomes concentrated in the urine, and sufficiently concentrated contrast material reduces the signal intensity of the urine due to T2* effects (Fig 5). This effect may be overcome with the use of low-dose gadolinium-based contrast material administration (as low as 0.01 mmol/kg), although such a technique does nothing to distend the collecting systems (14). Low-dose gadolinium-based contrast material administration has also been combined with oral hydration in an attempt to improve dilution and dispersion of excreted gadolinium-based contrast material throughout the collecting systems while improving ureteral distention (15). Unfortunately, MR urography performed with any amount of gadolinium-based contrast material without a pharmacologic means of enhancing urine flow tends to be suboptimal (16).
Figure 5a. T2* effect of concentrated gadolinium at excretory phase T1-weighted MR imaging. (a) Axial unenhanced T1-weighted MR image of the right kidney in an 85-year-old man with transitional cell carcinoma (TCC) shows a mass (arrows) in the renal collecting system. (b) On an axial gadolinium-enhanced excretory phase T1-weighted MR image obtained without hydration or diuretic administration, T2* effect (arrows) obscures the TCC.
Figure 5b. T2* effect of concentrated gadolinium at excretory phase T1-weighted MR imaging. (a) Axial unenhanced T1-weighted MR image of the right kidney in an 85-year-old man with transitional cell carcinoma (TCC) shows a mass (arrows) in the renal collecting system. (b) On an axial gadolinium-enhanced excretory phase T1-weighted MR image obtained without hydration or diuretic administration, T2* effect (arrows) obscures the TCC.
Diuretic administration can improve the quality of excretory MR urography by enhancing urine flow, resulting in dilution and uniform distribution of gadolinium-based contrast material throughout the urinary tract (17–19). One additional benefit of diuretic administration is expansion of the temporal window during which one may obtain images after gadolinium administration, since T2* effects become less limiting. A relatively low dose of furosemide on the order of 0.1 mg/kg (ie, 5–10 mg for adults) is typically used for MR urography provided no contraindications exist (1,3,20–22). For average-sized adults, we have found that a 5-mg dose of furosemide typically yields excellent image quality while permitting the patient to finish the examination without having to void. Symptoms of acute ureteral obstruction may be exacerbated by the administration of a diuretic, although such occurrences seem to be rare. In a report by Sudah et al (23), only one of 26 patients who presented with acute flank pain due to calculi developed exacerbation of symptoms after the administration of 0.1 mg/kg of furosemide for excretory MR urography. Contraindications for furosemide administration include anuria and hypersensitivity to furosemide, and electrolyte imbalance or hypotension should be corrected before administering furosemide. Patients who are allergic to sulfonamides may also be allergic to furosemide.
The optimal dose of gadolinium-based contrast material for diuretic-augmented MR urography has yet to be established. Nolte-Ernsting et al (1) advocated a gadolinium-based contrast material dose of 0.05 mmol/kg for diuretic-augmented excretory MR urography. Although doses of contrast material of less than 0.05 mmol/kg may yield satisfactory urographic images, concern exists that soft-tissue imaging will be compromised if the gadolinium dose is not sufficient.
The primary imaging sequence for excretory MR urography is the 3D gradient-echo sequence (3,23). Fat suppression enhances the conspicuity of the ureters and is recommended. Depending on the degree of background suppression desired, either a 3D soft-tissue imaging type sequence such as VIBE (volumetric interpolated breath-hold examination), FAME (fast acquisition with multiphase Efgre 3D), THRIVE (T1-weighted high-resolution isotropic volume examination), or liver acquisition with volume acceleration (LAVA) or a sequence normally used for MR angiography will suffice (Fig 6). Most modern imagers are capable of imaging the kidneys, ureters, and bladder in their entirety with a coronal 3D gradient-echo sequence during a single breath hold. Motion suppression is critical for MR urographic sequences, and breath-hold acquisitions have been shown to better demonstrate the pelvicaliceal systems compared with respiratory triggering (22). A coronal through-plane resolution of 2–4 mm is generally possible on newer imagers depending on the breath-holding ability of the patient (see Movie 3 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). For patients with a limited capacity to hold their breath, adequate spatial resolution can be achieved by imaging the urinary tract in segments. Imaging the urinary tract in segments with a smaller field of view and thinner sections also allows the acquisition of high-detail images of the collecting systems, although the degree of detail obtainable is limited by the signal-to-noise ratio (SNR). The use of echoplanar sequences for excretory MR urography has been described, although aside from reduced acquisition time, such techniques appear to offer few advantages over more conventional 3D gradient-echo techniques (22).
Figure 6a. Comparison of different sequences used for excretory MR urography. (a) On a coronal MIP image from excretory MR urographic data obtained with a 3D interpolated fat-suppressed gradient-echo sequence (LAVA) during breath holding, soft-tissue suppression is minimized owing to the use of a relatively low flip angle of 12°. (b) Coronal MIP image from excretory MR urographic data obtained with a 3D gradient-echo MR angiographic sequence shows improved background tissue suppression due in part to the use of a higher flip angle of 40°.
Figure 6b. Comparison of different sequences used for excretory MR urography. (a) On a coronal MIP image from excretory MR urographic data obtained with a 3D interpolated fat-suppressed gradient-echo sequence (LAVA) during breath holding, soft-tissue suppression is minimized owing to the use of a relatively low flip angle of 12°. (b) Coronal MIP image from excretory MR urographic data obtained with a 3D gradient-echo MR angiographic sequence shows improved background tissue suppression due in part to the use of a higher flip angle of 40°.
Excretory MR urography requires the excretion of gadolinium into the renal collecting systems to be effective. Therefore, excretory MR urography has no role in the evaluation of patients with severely compromised renal function and may require significantly delayed imaging in patients with urinary tract obstruction (Fig 4). In the case of a markedly dilated ureter, static-fluid MR urography is usually sufficient, although the use of gadolinium-based contrast material will occasionally help distinguish between high-grade partial and complete ureteral obstruction.
Comprehensive MR Urography Protocol
In many patients, static-fluid and excretory MR urography and conventional MR imaging sequences are complementary techniques, yielding different types of information that together can be helpful in establishing the correct diagnosis (24). A comprehensive MR urography protocol can be developed that facilitates evaluation of the renal parenchyma, upper urinary tracts, renal vasculature, urinary bladder, and surrounding structures (1,2). A comprehensive “one-stop shop” type of protocol, such as the one shown in the Table, can take between 30 and 60 minutes for an average technologist to complete depending on the choice of sequences and the available equipment. An abbreviated study that eliminates some components of the comprehensive examination can easily be completed in less than 30 minutes. We do not advocate our protocol as the only approach to a comprehensive MR urographic examination. Indeed, with the rapid pace of developments in the field of abdominal MR imaging, our own protocols are constantly evolving and change from year to year.
View this table:
Example of a Comprehensive 1.5-T MR Urography Protocol*
Hardware and Accessories
It would be impractical to address every possible commercially available hardware configuration in an article such as this one. Therefore, we will speak primarily from our own experience regarding hardware. Satisfactory MR urograms can be obtained at either 1.5 T or 3 T; we do not have experience performing MR urography at field strengths below 1.5 T. All studies described in this article were performed on a 1.5-T imager with an eight-channel phased-array torso coil unless otherwise specified. Although most of the newly developed torso coils allow coverage of the entire abdomen and pelvis in the axial plane with a single acquisition, we image the abdomen and pelvis separately using the maximum number of available coil elements for each acquisition to maximize SNR and to allow high-resolution breath-hold imaging. Most of the new, commercially available torso coils are compatible with sensitivity-encoding parallel imaging techniques. Use of parallel imaging reduces imaging time and the potential for respiratory motion artifacts. The improvement in image quality related to fewer respiratory artifacts usually more than compensates for the loss in SNR related to the use of parallel imaging. We limit our parallel imaging to acceleration factors of 2, since higher acceleration factors result in poor image quality on our current imagers. Mechanical compression has been used by some technologists to aid in urinary tract distention, although we have not found compression to be necessary (25).
Patient Preparation
Having patients void prior to entering the imager improves their comfort and prevents interruption of the study at an inopportune time. If no contraindications (eg, fluid restriction, congestive heart failure) exist, our patients are given 250 mL of normal saline solution intravenously at the start of imaging. Bowel contents are often bright with the T1- and T2-weighted sequences used for MR urography. We have found the use of oral negative contrast agents helpful in reducing the signal intensity of bowel contents, although the use of such agents is not required for MR urography. In most cases, imaging can be performed successfully with the patient supine.
Imaging Sequences
T2-weighted imaging can be performed with a variety of different sequences depending on the available equipment. For fat-suppressed T2-weighted imaging of the renal parenchyma and pelvic organs, we prefer a respiratory-triggered fast spin-echo sequence. For standard non-fat-suppressed T1-weighted imaging, in-phase and opposed-phase gradient-echo sequences can be useful for detecting intracellular lipid in incidental adrenal masses and clear cell carcinoma of the kidney as well as for characterizing some angiomyolipomas. For cine imaging of the ureters, a thick-slab, heavily T2-weighted single-shot fast spin-echo sequence similar to sequences used for MR cholangiopancreatography is performed. This sequence is typically performed 10–15 times with 5–10 seconds between acquisitions to prevent tissue saturation. The total number of thick-slab acquisitions can be varied to fit the circumstances.
For contrast material–enhanced T1-weighted imaging of the kidneys, a 3D interpolated fat-suppressed gradient-echo sequence combined with parallel imaging suffices. By obtaining pre- and postcontrast images using identical imaging parameters and respiratory cessation, a subtracted data set can be obtained that is useful for assessing the enhancement of solid masses. Acquiring a postcontrast data set during the arterial phase allows assessment of the renal arteries. After two postcontrast acquisitions, we immediately image through the urinary bladder to ensure that we obtain images with bladder wall enhancement prior to the arrival of gadolinium-based contrast material via the ureters (Fig 7). This procedure prevents mixing artifacts, which may obscure bladder tumors (Fig 8). Excretory phase images can be obtained approximately 5 minutes after contrast material injection in nonobstructed patients with normal or mildly impaired renal function. We routinely image the urinary tract in the axial and coronal planes during the excretory phase. It is beneficial to have patients raise the arms over the head during coronal imaging to prevent wraparound artifact.
Figure 7a. Low-grade papillary urothelial carcinoma in a 72-year-old man with hematuria. (a) Axial fat-suppressed gradient-echo T1-weighted MR image (LAVA) through the urinary bladder obtained after the administration of gadolinium-based contrast material but before excreted gadolinium had reached the bladder via the ureters clearly depicts a small enhancing tumor (arrow) against a dark background of unenhanced urine. (b) VR virtual cystoscopic image of the bladder created from the source images shows the small tumor (arrow) as well as a second larger tumor (arrowhead).
Figure 7b. Low-grade papillary urothelial carcinoma in a 72-year-old man with hematuria. (a) Axial fat-suppressed gradient-echo T1-weighted MR image (LAVA) through the urinary bladder obtained after the administration of gadolinium-based contrast material but before excreted gadolinium had reached the bladder via the ureters clearly depicts a small enhancing tumor (arrow) against a dark background of unenhanced urine. (b) VR virtual cystoscopic image of the bladder created from the source images shows the small tumor (arrow) as well as a second larger tumor (arrowhead).
Figure 8. Papillary urothelial carcinoma in a 68-year-old man who underwent MR urography for right-sided hydronephrosis. Axial gadolinium-enhanced fat-suppressed gradient-echo T1-weighted MR image (LAVA) through the urinary bladder shows an unsuspected bladder tumor (papillary urothelial carcinoma) (arrow) near the right ureterovesical junction. The tumor is almost obscured by enhanced urine that has entered the bladder and mixed with unenhanced urine. For this reason, a set of enhanced bladder images should be obtained prior to the arrival of gadolinium-based contrast material via the ureters.
Special Considerations
Pediatric Patients
The pediatric patient presents unique technical challenges for MR urography (26–30), including smaller physical size, inconsistent breath holding, and increased cardiac and respiratory rates (29). The majority of our pediatric patients are less than 6 years old and require sedation (27–29). Sedated patients can be successfully imaged during quiet respiration, although the use of respiratory-gated acquisitions has been described (31). Our pediatric MR urography protocol has evolved considerably over time. For excretory MR urography, we currently hydrate patients with 10 mL/kg of normal saline solution and administer furosemide at a dose of 0.1 mg/kg up to a maximum of 5 mg prior to the administration of gadolinium-based contrast material (standard dose of 0.1 mmol/kg). In pediatric patients with high-grade obstructions, static-fluid MR urography can be used to assess nonfunctioning systems. Static-fluid MR urography has a distinct advantage over excretory urography, which routinely presents problems in documenting the course and insertion of ureters when there is obstruction or poor function (26,28–30). In pediatric patients, performing dynamic contrast-enhanced imaging in the coronal plane allows improved assessment of vascular structures, such as crossing vessels in the setting of UPJ obstruction (28). This approach also allows contemporaneous imaging of the kidneys, ureters, and bladder, given the small size of many pediatric patients. Time–signal-intensity curves have been successfully used to assess renal obstruction in an effort to duplicate the curves generated with diuretic-enhanced renal scintigraphy, although the generation of curves based on segmentation of the renal cortex and medulla may be time consuming in the absence of software automation (32–34). Preliminary studies have also shown the potential of MR urography to suggest the diagnosis of vesicoureteral reflux on the basis of time–signal-intensity curves generated from diuretic-augmented excretory MR urographic images obtained over a period of 40 minutes (35).
Pregnant Patients
Contrast-enhanced MR urography is generally unnecessary in pregnant women. Instead, T2-weighted (static-fluid) urography is performed. Multiple acquisitions (cine MR urography) may be necessary to visualize the entire ureters and exclude fixed narrowings or filling defects. In the latter stages of pregnancy, imaging with the patient in the left lateral decubitus position helps reduce pressure exerted on the inferior vena cava by the gravid uterus. Roy et al (36) reported excellent results with T2-weighted MR urography in identifying urinary tract dilatation and level of obstruction in 17 pregnant patients. The challenge of interpreting MR urographic images obtained during pregnancy remains the differentiation of physiologic hydronephrosis from pathologic obstruction (36–38). The MR urographic findings of physiologic hydronephrosis that have been described include compression of the mid-ureter with tapering at the pelvic brim and no discernable filling defect (Fig 9). Tapering at another level suggests an alternative diagnosis, such as ureteral stone. The ureter below the level of compression should be relatively collapsed, although this segment can be seen to intermittently fill and empty at cine urography. A standing column of urine between the site of physiologic compression and the ureterovesical junction suggests the presence of a distal ureteral stone. In cases of acute calculous obstruction, renal and perirenal edema are often present.
Figure 9a. Physiologic hydronephrosis in a 28-year-old woman late in the 2nd trimester of pregnancy. (a) Thick-slab static-fluid MR urogram shows tapering of the right ureter at the pelvic brim (arrow). (b) Sagittal single-shot fast spin-echo MR image shows gradual smooth tapering of the right ureter (arrow) at the site of compression between the gravid uterus and the psoas muscle.
Figure 9b. Physiologic hydronephrosis in a 28-year-old woman late in the 2nd trimester of pregnancy. (a) Thick-slab static-fluid MR urogram shows tapering of the right ureter at the pelvic brim (arrow). (b) Sagittal single-shot fast spin-echo MR image shows gradual smooth tapering of the right ureter (arrow) at the site of compression between the gravid uterus and the psoas muscle.
Renal Insufficiency
The success of static-fluid MR urography depends on the presence of fluid within the urinary collecting system irrespective of renal function. Any patient who can undergo MR imaging can potentially undergo static-fluid MR urography, although the latter may be of limited value for nondilated collecting systems. The success of excretory MR urography depends on the excretion of gadolinium into the renal collecting systems. Consequently, patients with severely compromised renal function are poor candidates for excretory MR urography. In the past, excretory MR urography has been advocated for use in patients with less severe renal insufficiency as a means of avoiding the use of iodinated contrast material, given the reported low nephrotoxicity of gadolinium chelates at standard clinical doses (39–42). Relatively recent reports linking gadolinium administration to a disorder known as nephrogenic systemic fibrosis have resulted in new recommendations to avoid (whenever possible) the use of gadolinium-based contrast material in patients with moderate to severe renal insufficiency (43–48). It is important to note that the factors contributing to the development of nephrogenic systemic fibrosis remain an area of intense investigation, and physicians are encouraged to stay abreast of new developments and recommendations regarding the use of gadolinium-based contrast material in patients with renal insufficiency.
Imaging at 3 T
MR urography can be successfully performed at 3 T (Fig 10). The improved SNR available at 3 T allows contiguous imaging from the top of the kidneys through the pelvis without switching coil configurations. We are routinely able to image the entire urinary tract in the coronal plane with an excellent SNR on our 3-T imager using partitions of 2 mm (see Movie 4 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). To our knowledge, there have been no direct comparisons between 1.5-T and 3-T MR urography. Therefore, it remains to be seen whether 3-T imaging offers significant advantages in terms of lesion detection. Individuals performing MR urography at 3 T should be aware of the potential limitations of abdominal and pelvic imaging performed at higher field strengths (49). Prolongation of the T1 relaxation time could potentially have a negative impact on image contrast and lesion conspicuity on T1-weighted images, and chemical shift and susceptibility artifacts are accentuated at higher field strengths. Standing wave and conductivity artifacts may also degrade the quality of images obtained with sequences that are often performed for MR urography (eg, single-shot fast spin-echo) (49).
Figure 10. MR urography at 3 T in a 55-year-old man with hematuria. Coronal MIP image from diuretic-augmented excretory MR urographic data obtained after the administration of 0.05 mmol/kg of gadobenate dimeglumine shows normal collecting systems and excellent image quality over a large field of view.
Clinical Applications
Urolithiasis
In the United States, most patients with acute symptoms thought to be related to urolithiasis undergo unenhanced multidetector CT rather than MR urography. Regardless, calculi are common and will likely be encountered on occasion by anyone performing MR urography. Most urinary tract calculi appear as signal voids with T1-and T2-weighted sequences. At both static-fluid and excretory MR urography, calculi appear as filling defects when surrounded by urine or contrast material (Figs 11, 12). However, low-signal-intensity filling defects in the urinary tract are not specific for calculi. The most common causes of noncalculous filling defects within the urinary tract at static-fluid MR urography are blood clots and tumor. Urinary tract calculi can often be distinguished from blood clots, since the latter typically exhibit high-signal-intensity elements on unenhanced T1-weighted images (Fig 13). A calculus can also usually be distinguished from neoplasm, since the latter typically enhances after intravenous contrast material administration. MR imaging findings of acute stone colic include increased perinephric fluid on T2-weighted images, ureteral dilatation proximal to the stone, and a filling defect on T2-weighted or excretory MR urograms. The presence of perirenal fluid can be helpful in distinguishing acute from chronic ureteric obstruction (8). Sudah et al (23) demonstrated improved sensitivity for ureteral calculi for diuretic-augmented excretory MR urography (96.2%–100%) compared with a T2-weighted technique (53.8%–57.7%). In their study, perirenal high signal intensity was present on T2-weighted images in 92% of patients with ureteral calculi (23). Karabacakoglu et al (21) successfully found 26 of 28 collecting system calculi with diuretic-augmented excretory MR urography.
Figure 11a. Urinary tract calculi in a 62-year-old man with hematuria. (a) Coronal 3D fat-suppressed gradient-echo MR image (LAVA) obtained as part of an excretory MR urographic study shows small filling defects (arrow) within the right renal pelvis, findings that represent calculi. (b) Unenhanced CT scan shows calculi in the right renal pelvis (arrow). Arrowhead indicates a caliceal stone, which could not be seen even in retrospect on MR images.
Figure 11b. Urinary tract calculi in a 62-year-old man with hematuria. (a) Coronal 3D fat-suppressed gradient-echo MR image (LAVA) obtained as part of an excretory MR urographic study shows small filling defects (arrow) within the right renal pelvis, findings that represent calculi. (b) Unenhanced CT scan shows calculi in the right renal pelvis (arrow). Arrowhead indicates a caliceal stone, which could not be seen even in retrospect on MR images.
Figure 12. Nonobstructing ureteral calculus in a 42-year-old woman. Coronal MIP image from 3-T excretory MR urographic data shows a small filling defect (arrow) in the midportion of the left ureter. Minimal left-sided hydronephrosis is also seen. Note that contrast material is present within the ureter below the level of the calculus. These findings were confirmed with unenhanced CT and retrograde urography.
Figure 13a. Blood clots in a 90-year-old man with hematuria. (a) Unenhanced fat-suppressed gradient-echo 3-T T1-weighted MR image obtained prior to voiding shows a high-signal-intensity blood clot (arrow) in the bladder. (b) On a postvoiding image, the blood clot is gone. The voided urine contained a large volume of clotted blood.
Figure 13b. Blood clots in a 90-year-old man with hematuria. (a) Unenhanced fat-suppressed gradient-echo 3-T T1-weighted MR image obtained prior to voiding shows a high-signal-intensity blood clot (arrow) in the bladder. (b) On a postvoiding image, the blood clot is gone. The voided urine contained a large volume of clotted blood.
A variety of studies have addressed the detection of urinary tract calculi with MR urography compared with other modalities. Regan et al (8) compared a combination of static-fluid MR urography and abdominal radiography with unenhanced spiral CT and found that the former was more sensitive in the detection of secondary findings of acute ureteral obstruction such as perirenal fluid and ureteric dilatation, although MR urography combined with abdominal radiography showed only 72% of the ureteral calculi seen at CT. In a study by Jung et al (50), diuretic-augmented excretory MR urography helped make the correct diagnosis in 64 of 72 patients with ureteric calculi, compared with 49 of 72 patients for conventional intravenous urography. In two patients with calculi in this study, an incorrect diagnosis of tumor was made at MR urography (50). In a study of 149 patients with ureteral obstruction, MR urography was significantly inferior to unenhanced CT in identifying the site of stone impaction (sensitivity, 69% vs 100%) but was superior to unenhanced CT in identifying ureteral strictures (83% vs 28%) and neoplastic obstruction (42 of 43 neoplasms vs 22 of 43) (51). In this study, the sensitivity of unenhanced CT for noncalculous obstruction was 40%, compared with 89% sensitivity for MR urography and 18% for a combination of KUB (kidneys, ureters, and bladder) and ultrasonography (US) (51).
Urinary Tract Obstruction Unrelated to Urolithiasis
MR urography is more sensitive and specific for noncalculous urinary tract obstruction than is unenhanced CT (51,52). Benign strictures of the ureter may complicate abdominal and pelvic inflammatory processes (eg, appendicitis, Crohn disease, endometriosis), infection (eg, tuberculosis), radiation therapy, surgical or interventional procedures, or stone disease. Benign strictures of the ureter are typically smoothly tapering and not associated with a soft-tissue mass (Fig 14). Cine or excretory MR urography is helpful in gauging the severity of a stricture. In cases of partial obstruction, cine MR urography will demonstrate intermittent distention and collapse of the ureter below the level of narrowing, whereas excretory MR urography will demonstrate contrast enhancement of the ureter distal to the narrowing (see Movie 5 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). High-grade obstruction will result in delayed excretion of gadolinium-based contrast material on the affected side. Ureteral jets can also be demonstrated with cine urography in the absence of ureteral obstruction.
Figure 14a. High-grade inflammatory stricture in a 68-year-old man with hydronephrosis. (a) Coronal static-fluid MR urogram shows smoothly contoured obstruction of the right ureter (arrow) associated with debris. (b) Coronal single-shot fast spin-echo MR image shows smoothly tapering obstruction of the right ureter (arrow) with no evidence of an associated soft-tissue mass. The patient had a history of complicated appendicitis, which likely accounted for the stricture. No tumor was identified at ureteroscopy.
Figure 14b. High-grade inflammatory stricture in a 68-year-old man with hydronephrosis. (a) Coronal static-fluid MR urogram shows smoothly contoured obstruction of the right ureter (arrow) associated with debris. (b) Coronal single-shot fast spin-echo MR image shows smoothly tapering obstruction of the right ureter (arrow) with no evidence of an associated soft-tissue mass. The patient had a history of complicated appendicitis, which likely accounted for the stricture. No tumor was identified at ureteroscopy.
Extrinsic narrowing of the ureters may occur with entities such as uterine fibroids, fluid collections, retroperitoneal fibrosis, and vascular abnormalities (Fig 15). Benign extrinsic processes may cause deviation of one or both ureters and often result in smooth tapering of the ureter at the site of compression. As with other modalities, retroperitoneal fibrosis can result in medial deviation of the ureters at MR urography. Benign extrinsic compression of the ureter rarely results in complete obstruction.
Figure 15a. Extrinsic compression of the ureter in a 39-year-old woman with mild renal insufficiency and hydronephrosis of a solitary right kidney. (a) Coronal MIP image from excretory MR urographic data shows a smoothly tapering site of partial ureteral obstruction in the pelvis (arrow). (b) Oblique coronal image reconstructed from axial 3D excretory phase fat-suppressed gradient-echo pelvic imaging data shows compression of the right ureter (arrow) as it passes between the right external iliac artery and vein (a rare anatomic variant).
Figure 15b. Extrinsic compression of the ureter in a 39-year-old woman with mild renal insufficiency and hydronephrosis of a solitary right kidney. (a) Coronal MIP image from excretory MR urographic data shows a smoothly tapering site of partial ureteral obstruction in the pelvis (arrow). (b) Oblique coronal image reconstructed from axial 3D excretory phase fat-suppressed gradient-echo pelvic imaging data shows compression of the right ureter (arrow) as it passes between the right external iliac artery and vein (a rare anatomic variant).
Neoplastic obstruction of the urinary tract can result from benign or malignant processes. Benign urothelial tumors such as fibroepithelial polyps appear as filling defects at MR urography (53). Malignant processes that can obstruct the ureters include intrinsic urothelial tumors such as TCC of the ureters or bladder, metastatic tumors to the ureters or periureteric tissues, neoplastic lymph nodes, and direct invasion from extraureteral neoplasms (Figs 1, 16). When neoplastic obstruction is suspected, it is important to include sequences to evaluate the soft tissues in addition to MR urographic sequences optimized to visualize the lumen of the urinary tract. Tumor that arises directly from or invades the ureter often results in irregularity of the ureter at the point of obstruction or appears as an irregular filling defect. Unlike calculi, most neoplasms will enhance after intravenous contrast material administration.
Figure 16a. Malignant obstruction of the ureter in a 74-year-old woman with recurrent leiomyosarcoma. (a) Coronal static-fluid MR urogram shows abrupt irregular obstruction of the left distal ureter (arrow). (b) Coronal single-shot fast spin-echo MR image shows a soft-tissue mass (arrow) at the site of ureteral obstruction. Subsequent biopsy revealed recurrent leiomyosarcoma (cf Fig 12).
Figure 16b. Malignant obstruction of the ureter in a 74-year-old woman with recurrent leiomyosarcoma. (a) Coronal static-fluid MR urogram shows abrupt irregular obstruction of the left distal ureter (arrow). (b) Coronal single-shot fast spin-echo MR image shows a soft-tissue mass (arrow) at the site of ureteral obstruction. Subsequent biopsy revealed recurrent leiomyosarcoma (cf Fig 12).
The role of MR urography for screening patients at risk for urothelial malignancy has yet to be defined. In one study, static-fluid MR urography performed with a high-resolution technique in two planes through the level of ureteral obstruction was successful in demonstrating eight ureteral and five renal pelvic TCCs in 23 high-risk patients who were poor candidates for other types of imaging examinations (54).
Bladder cancer, cervical cancer, and prostate cancer are relatively common causes of malignant ureteral obstruction (Fig 1). Most malignant tumors of the urinary epithelium are TCCs (Fig 17). TCCs can appear as sessile filling defects or wall thickening (55). Proximal ureteral dilatation may be present. As with other forms of urography, the “goblet” sign can occasionally be seen at MR urography in the setting of TCC of the ureter (3). Urethelial tumors generally have intermediate signal intensity at MR imaging and demonstrate enhancement that is not present with calculi or blood clots (53). It is important to evaluate the entire urinary tract in the setting of TCC, given the propensity of TCC for multifocal involvement (Fig 18).
Figure 17. TCC in an 85-year-old man. Coronal partial-volume MIP image from excretory MR urographic data shows a right lower pole mass (arrow) extending into the renal pelvis.
Figure 18a. Multifocal TCC in an 82-year-old man. (a) Coronal MIP image from 3-T excretory MR urographic data shows tumors of the left distal ureter (arrow) and left upper pole renal collecting system (arrowhead). (b) Axial gadolinium-enhanced fat-suppressed gradient-echo T1-weighted MR image through the bladder obtained prior to the arrival of contrast material via the ureters clearly depicts a mass protruding from the left distal ureter (arrow).
Figure 18b. Multifocal TCC in an 82-year-old man. (a) Coronal MIP image from 3-T excretory MR urographic data shows tumors of the left distal ureter (arrow) and left upper pole renal collecting system (arrowhead). (b) Axial gadolinium-enhanced fat-suppressed gradient-echo T1-weighted MR image through the bladder obtained prior to the arrival of contrast material via the ureters clearly depicts a mass protruding from the left distal ureter (arrow).
Hematuria
The evaluation of hematuria with MR imaging requires the use of routine imaging sequences in addition to MR urography. This approach facilitates the detection of renal parenchymal and vascular lesions as well as urothelial abnormalities (Fig 19). MR imaging cannot currently match the spatial resolution of CT, although it is excellent for the detection, characterization, and staging of renal neoplasms (56–60). Small nonobstructive calculi and calcifications will likely be missed at MR urography performed for the evaluation of hematuria. Furthermore, the actual sensitivity of MR urography for the detection of small urothelial neoplasms is unknown.
Figure 19a. Arteriovenous fistula in a 67-year-old man with hematuria. (a) Coronal partial-volume MIP image from excretory MR urographic data shows the left proximal ureter with a corkscrew appearance (arrow). (b) Coronal partial-volume MIP image from axial contrast-enhanced 3D early venous phase gradient-echo MR data shows an enlarged periureteral vein (arrow), a finding that accounts for the abnormal course of the left ureter. Review of the patient’s medical records revealed prior left lower pole renal biopsy, which likely accounted for the arteriovenous fistula.
Figure 19b. Arteriovenous fistula in a 67-year-old man with hematuria. (a) Coronal partial-volume MIP image from excretory MR urographic data shows the left proximal ureter with a corkscrew appearance (arrow). (b) Coronal partial-volume MIP image from axial contrast-enhanced 3D early venous phase gradient-echo MR data shows an enlarged periureteral vein (arrow), a finding that accounts for the abnormal course of the left ureter. Review of the patient’s medical records revealed prior left lower pole renal biopsy, which likely accounted for the arteriovenous fistula.
Congenital Anomalies
MR urography can be used to evaluate patients with absent kidney, abnormally positioned or rotated kidney, renal duplication, renal dysplasia, ectopic ureter, retrocaval ureter, primary megaureter, or UPJ obstruction (Fig 20) (26–30,53). In our practice, complicated renal duplication and congenital UPJ obstruction constitute the two most common indications in this category.
Figure 20. Horseshoe kidney and congenital UPJ stenosis in a 67-year-old woman. VR image from 3-T excretory MR urographic data shows a dilated left moiety collecting system to the level of the UPJ (arrow).
Renal duplication can be partial, with the ureters joining above the bladder, or complete, with two ureters inserting separately on one side. Complete or complicated (eg, obstructed moiety) renal duplication is more common in females than in males, and MR urography has been shown to be superior to intravenous urography and US in evaluating the complicated duplex kidney (12,26,28). In cases of complete duplication, the upper pole ureter typically inserts inferior and medial to the lower pole ureter and is more prone to obstruction (Fig 21). The upper pole ureter can insert either ectopically, with development of a ureterocele, or in an extravesicular location. Excretion function of the kidney is not a limiting factor in the diagnosis of an ectopic ureter, since static-fluid MR urographic images are usually sufficient (26,28–30). The lower pole ureter of a duplicated kidney has a tendency to demonstrate reflux, although this phenomenon may be difficult to appreciate at MR urography performed only to evaluate the anatomy.
Figure 21a. Duplicated right renal collecting system in a 7-month-old girl. (a) Coronal static-fluid MR urogram shows a dilated right upper pole moiety (top arrow) and an ectopic ureter (bottom arrow) extending below the bladder. (b) Axial fat-suppressed T2-weighted MR image of the pelvis shows the ectopic ureter (arrow) extending below the bladder base to insert on a sidewall of the vagina (arrowhead). Note the high-signal-intensity urine within the vagina.
Figure 21b. Duplicated right renal collecting system in a 7-month-old girl. (a) Coronal static-fluid MR urogram shows a dilated right upper pole moiety (top arrow) and an ectopic ureter (bottom arrow) extending below the bladder. (b) Axial fat-suppressed T2-weighted MR image of the pelvis shows the ectopic ureter (arrow) extending below the bladder base to insert on a sidewall of the vagina (arrowhead). Note the high-signal-intensity urine within the vagina.
The UPJ is the most common site of urinary tract obstruction in children. Historically, intravenous urography and lasix (furosemide) renography have been the examinations of choice for the assessment and grading of obstruction, although MR urography can provide both the anatomic information of intravenous urography and the functional data of renal scintigraphy in a single test without exposure to ionizing radiation (28–30,61). A variety of theories have been proposed to explain the etiology of UPJ obstruction, including abnormal smooth muscle arrangement, abnormal innervation of the ureter, crossing vessel, and fibrotic scar. The prognosis for a patient with congenital UPJ obstruction is best predicted on the basis of renal function, and declining function is cause for intervention. In older children, up to 50% of symptomatic UPJ obstructions will be associated with a crossing vessel, the presence of which alters the surgical approach (Fig 22) (29). Therefore, including an MR angiographic sequence is advisable when assessing for UPJ obstruction.
Figure 22a. Left UPJ obstruction in a 15-year-old girl. (a) Coronal static-fluid MR urogram shows a dilated left renal pelvis (arrow). (b) Oblique coronal reformatted image from MR angiographic data obtained as part of an MR urographic examination shows a crossing vessel (arrow) at the site of a left UPJ obstruction.
Figure 22b. Left UPJ obstruction in a 15-year-old girl. (a) Coronal static-fluid MR urogram shows a dilated left renal pelvis (arrow). (b) Oblique coronal reformatted image from MR angiographic data obtained as part of an MR urographic examination shows a crossing vessel (arrow) at the site of a left UPJ obstruction.
Pre- and Postoperative Assessment
MR urography is easily combined with MR angiography and standard MR imaging for the pre-operative assessment of the arterial supply, collecting system, and renal parenchyma in potential renal transplant donors (25). Likewise, MR imaging can be used to evaluate the renal vasculature, renal parenchyma, collecting system, and peritransplant tissues in renal transplant recipients in a single test, making it a useful adjunct to US, which will likely remain the first-line imaging examination for renal transplant patients (Fig 23) (62–64). When examining renal transplants with MR urography, it is advantageous to combine static-fluid with excretory phase sequences, provided sufficient renal function is present. Schubert et al (65) studied nine renal transplant recipients with static-fluid MR urography and found the lack of functional information to be limiting. In their small series, static-fluid MR urography revealed a definite cause of obstruction in only two of five cases (65). Static-fluid MR urography may not be capable of helping to distinguish between ureteral obstruction and vesicoureteral reflux in some cases, and ureteral leak cannot be demonstrated in the absence of excreted contrast material. Excretory MR urography provides some functional information, and findings are not likely to be obscured by peritransplant fluid collections, which are relatively common after kidney transplantation. MR imaging can also be used to demonstrate postoperative anatomy or to screen for complications after urinary diversion or neobladder reconstruction (Figs 24, 25; see also Movie 6 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1) (24,66).
Figure 23. MR urography in a 44-year-old man with a left iliac fossa renal transplant and hematuria. Coronal MIP image from 3-T excretory MR urographic data shows the transplanted kidney with a normal collecting system.
Figure 24a. Ureteral stricture after ileal conduit reconstruction in a 68-year-old man. (a) Coronal MIP image from excretory MR urographic data shows an ileal conduit (arrow) that was created after cystectomy for TCC of the bladder. Note the dilated right collecting system (arrowhead). A standing column of urine was seen in the right ureter at cine MR urography (see Movie 6 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). (b) Oblique coronal VR image from excretory MR urographic data shows that an anastomotic stricture (arrow) is responsible for the dilated right collecting system. The patient subsequently underwent successful balloon dilation of the stricture.
Figure 24b. Ureteral stricture after ileal conduit reconstruction in a 68-year-old man. (a) Coronal MIP image from excretory MR urographic data shows an ileal conduit (arrow) that was created after cystectomy for TCC of the bladder. Note the dilated right collecting system (arrowhead). A standing column of urine was seen in the right ureter at cine MR urography (see Movie 6 at radiographics.rsnajnls.org/cgi/content/full/28/1/23/DC1). (b) Oblique coronal VR image from excretory MR urographic data shows that an anastomotic stricture (arrow) is responsible for the dilated right collecting system. The patient subsequently underwent successful balloon dilation of the stricture.
Figure 25a. Neobladder outlet obstruction in a 65-year-old man who had undergone cystectomy and ileal neobladder reconstruction for adenocarcinoma of the bladder. (a) Coronal MIP image from excretory MR urographic data obtained as part of routine follow-up demonstrates marked dilatation of both upper collecting systems (arrows) and the neobladder due to outlet obstruction. The patient was asymptomatic at the time of the examination. (b) Axial gadolinium-enhanced 3D fat-suppressed gradient-echo MR image (LAVA) through the urethral anastomosis shows that an enhancing recurrent tumor (arrow) is responsible for the outlet obstruction.
Figure 25b. Neobladder outlet obstruction in a 65-year-old man who had undergone cystectomy and ileal neobladder reconstruction for adenocarcinoma of the bladder. (a) Coronal MIP image from excretory MR urographic data obtained as part of routine follow-up demonstrates marked dilatation of both upper collecting systems (arrows) and the neobladder due to outlet obstruction. The patient was asymptomatic at the time of the examination. (b) Axial gadolinium-enhanced 3D fat-suppressed gradient-echo MR image (LAVA) through the urethral anastomosis shows that an enhancing recurrent tumor (arrow) is responsible for the outlet obstruction.
Pitfalls and Artifacts
As with any MR imaging technique, one must be aware of potential pitfalls when interpreting findings at MR urography (67). When reviewing MR urographic images created with MIP or VR algorithms, one should always consult the original data (source images) to ensure that small filling defects are not obscured by surrounding high-signal-intensity urine. Thick-slab acquisitions may also mask filling defects and should be used primarily to document the presence and level of obstruction (Fig 26).
Figure 26. Papillary urothelial carcinoma of the bladder in the same patient as in Figure 14a. VR virtual cystoscopic image shows an unsuspected polypoid tumor (arrow). The tumor could not be seen even in retrospect on thick-slab static-fluid MR urograms regardless of the window width and level settings used (cf Fig 14a). This finding demonstrates that even sizable filling defects can be obscured at thick-slab MR urography that encompasses the entire urinary tract.
Whereas small intrarenal calculi are usually inconspicuous at MR imaging, large calculi can mimic a dilated, poorly functioning collecting system on T1-weighted images (Fig 27). However, this pitfall is easily avoided because urine is typically bright and calculi are typically dark on unenhanced T2-weighted images. Of course, correlation of the MR urographic findings with other available imaging findings such as radiographic or CT findings is always a good idea. Another mimic of a dilated intrarenal collecting system is the renal sinus cyst (Fig 28). When viewed on T1- or T2-weighted images obtained prior to the intravenous administration of contrast material, renal sinus cysts have the same signal intensity as urine. Therefore, renal sinus cysts are best differentiated from hydronephrosis on postcontrast excretory phase images.
Figure 27a. Obstructing staghorn calculus in a 51-year-old patient. (a) Axial gadolinium-enhanced 3D fat-suppressed gradient-echo MR image through the kidneys shows bilateral signal voids in the region of the collecting systems (arrows) and enhancing urothelium in the left kidney. It is difficult to differentiate between hydronephrosis and a renal calculus on this image. (b) Coronal single-shot fast spin-echo MR image through the kidneys obtained prior to the administration of gadolinium-based contrast material reveals a low-signal-intensity area in the left renal collecting system (arrow), a finding that represents a staghorn calculus associated with a hydrocalix (arrowhead). A large calculus was also visible on the patient’s right side on other images.
Figure 27b. Obstructing staghorn calculus in a 51-year-old patient. (a) Axial gadolinium-enhanced 3D fat-suppressed gradient-echo MR image through the kidneys shows bilateral signal voids in the region of the collecting systems (arrows) and enhancing urothelium in the left kidney. It is difficult to differentiate between hydronephrosis and a renal calculus on this image. (b) Coronal single-shot fast spin-echo MR image through the kidneys obtained prior to the administration of gadolinium-based contrast material reveals a low-signal-intensity area in the left renal collecting system (arrow), a finding that represents a staghorn calculus associated with a hydrocalix (arrowhead). A large calculus was also visible on the patient’s right side on other images.
Figure 28a. Renal sinus cyst mimicking hydronephrosis. (a) Axial fat-suppressed T2-weighted MR image through the left kidney demonstrates fluid signal intensity (arrow) in the region of the left renal pelvis, a finding that might be confused with hydronephrosis. (b) Axial gadolinium-enhanced 3D fat-suppressed excretory phase gradient-echo MR image through the kidneys shows enhancement of the left proximal ureter (arrow) and no enhancement in the region of the left renal pelvis, findings that exclude hydronephrosis and indicate that the area of increased signal intensity on the T2-weighted image represents a renal sinus cyst.
Figure 28b. Renal sinus cyst mimicking hydronephrosis. (a) Axial fat-suppressed T2-weighted MR image through the left kidney demonstrates fluid signal intensity (arrow) in the region of the left renal pelvis, a finding that might be confused with hydronephrosis. (b) Axial gadolinium-enhanced 3D fat-suppressed excretory phase gradient-echo MR image through the kidneys shows enhancement of the left proximal ureter (arrow) and no enhancement in the region of the left renal pelvis, findings that exclude hydronephrosis and indicate that the area of increased signal intensity on the T2-weighted image represents a renal sinus cyst.
Susceptibility artifact from metallic objects such as surgical clips can interfere with the visualization of ureteral segments or create the appearance of a ureteral stenosis. As with renal calculi, correlation with radiographic or CT findings can be helpful, although most regions of susceptibility artifact can be correctly identified on gradient-echo source images. Air from recent intervention or an indwelling nephrostomy tube may result in filling defects that simulate calculi.
Flow-related artifacts are relatively common with single-shot fast spin-echo sequences (eg, half-Fourier rapid acquisition with relaxation enhancement, single-shot turbo spin-echo). With such sequences, fluid in motion will demonstrate varying degrees of signal intensity loss that may simulate filling defects within the collecting systems (Fig 29). Girish et al (68) reported filling defect artifacts at heavily T2-weighted diuretic-augmented imaging in 23 of 45 patients with neurogenic bladder dysfunction, with the majority of these artifacts seen in the pelvicaliceal system and upper ureters. The authors did not identify the cause of these artifacts; however, the artifacts were likely caused by a variety of processes, including flow. In our experience, flow-related artifacts tend to be transient or change appearance between sequences. Unlike true filling defects, flow-related artifacts will not have a corresponding abnormality at excretory MR urography.
Figure 29a. Flow-related artifact versus tumor. (a) Coronal single-shot fast spin-echo MR image obtained in a 91-year-old man without urinary tract symptoms shows an intermediate-signal-intensity “mass” (arrow) in the base of the bladder. This finding did not persist with other imaging sequences or on other sections from the same sequence and represents the typical appearance of a flow-related artifact. (b) Coronal single-shot fast spin-echo MR image obtained in a 72-year-old man with hematuria shows an intermediate-signal-intensity mass (arrow) in the bladder base. The mass persisted with all sequences and represents TCC. Note the similarity to the flow-related artifact seen in a.
Figure 29b. Flow-related artifact versus tumor. (a) Coronal single-shot fast spin-echo MR image obtained in a 91-year-old man without urinary tract symptoms shows an intermediate-signal-intensity “mass” (arrow) in the base of the bladder. This finding did not persist with other imaging sequences or on other sections from the same sequence and represents the typical appearance of a flow-related artifact. (b) Coronal single-shot fast spin-echo MR image obtained in a 72-year-old man with hematuria shows an intermediate-signal-intensity mass (arrow) in the bladder base. The mass persisted with all sequences and represents TCC. Note the similarity to the flow-related artifact seen in a.
Hemorrhage into the renal collecting systems may appear bright on gradient-echo T1-weighted images and may be obscured by gadolinium-based contrast material. Therefore, we always perform precontrast gradient-echo T1-weighted imaging in at least one plane. Hemorrhage can also potentially interfere with static-fluid MR urography by reducing the signal intensity of urine. Decreasing the echo time at T2-weighted imaging may help overcome this limitation to some extent. Ureteral peristalsis may occasionally result in ghost artifacts on 3D gradient-echo images, although these artifacts rarely interfere substantially with interpretation (22).
Conclusions
When properly performed, MR urography can be a valuable means of noninvasively assessing the urinary tract. Static-fluid and excretory MR urography can be combined with conventional MR imaging to provide a comprehensive evaluation of the kidneys, ureters, bladder, vasculature, and soft tissues in patients with symptoms referable to the urinary tract. T2-weighted techniques are excellent for demonstrating dilated or obstructed collecting systems, whereas excretory MR urography provides excellent visualization of nonobstructed systems. MR urography can be useful for assessing patients with a variety of urinary tract disorders and allows the evaluation of pediatric and pregnant patients without the use of ionizing radiation. The successful interpretation of MR urographic examinations requires familiarity with the numerous potential pitfalls and artifacts that may be encountered.
Langganan:
Postingan (Atom)